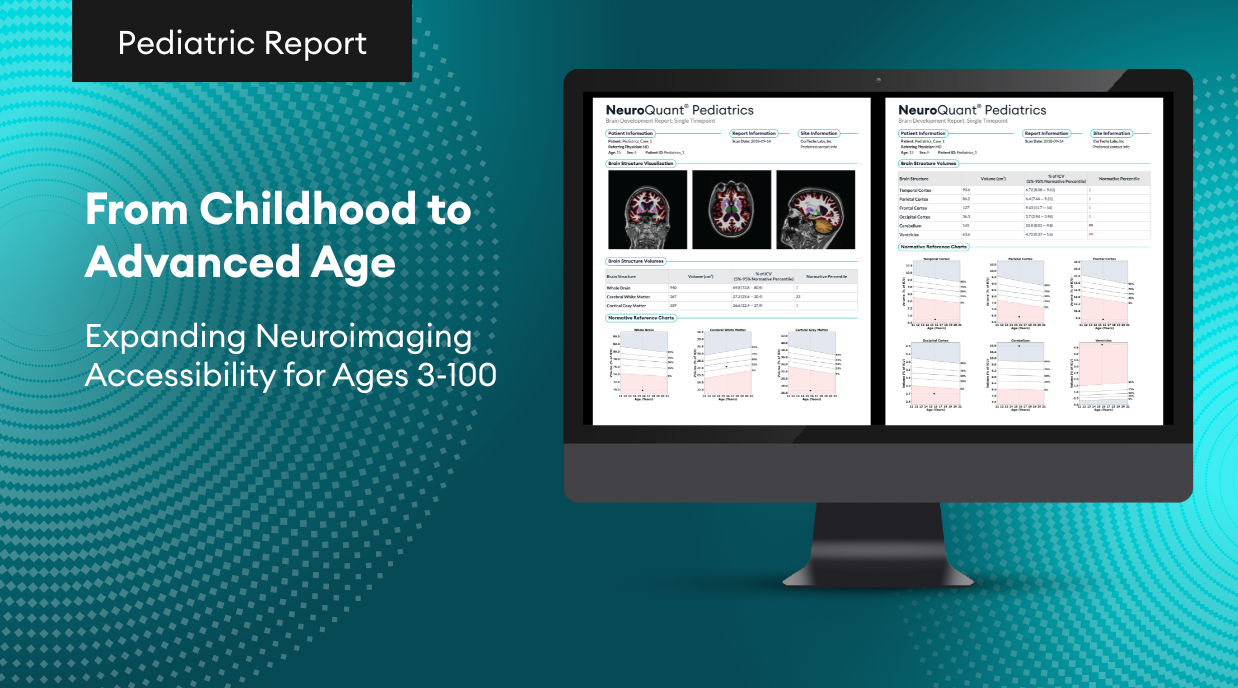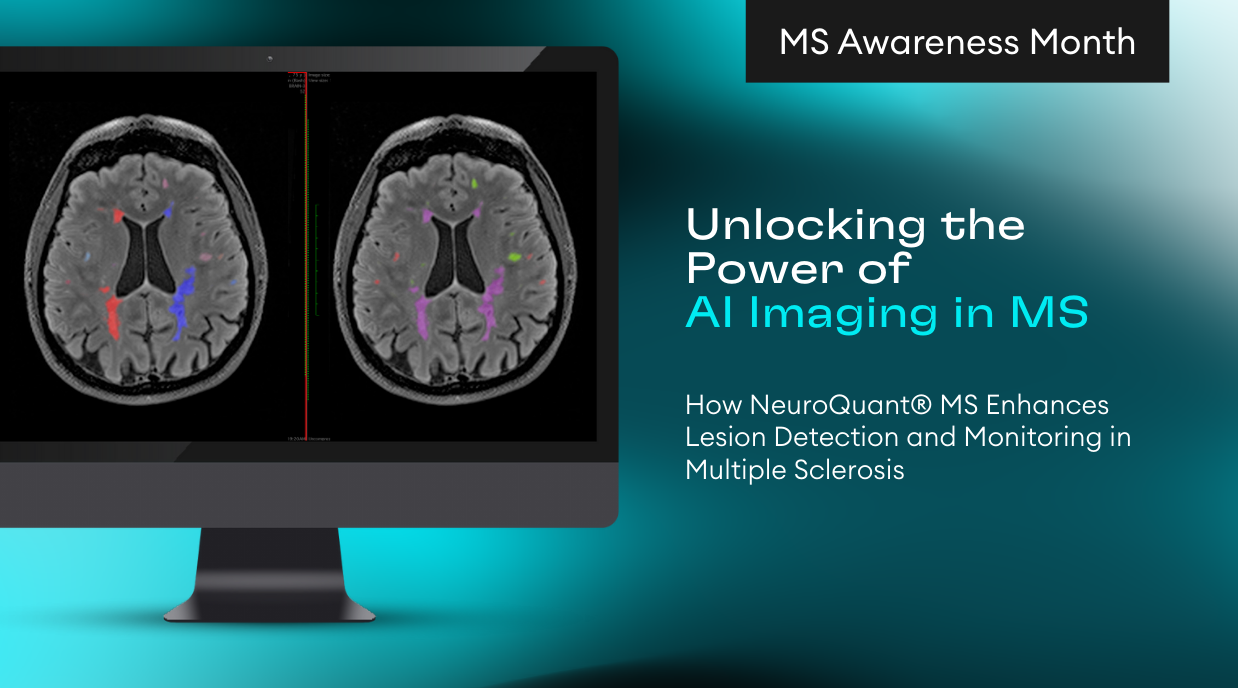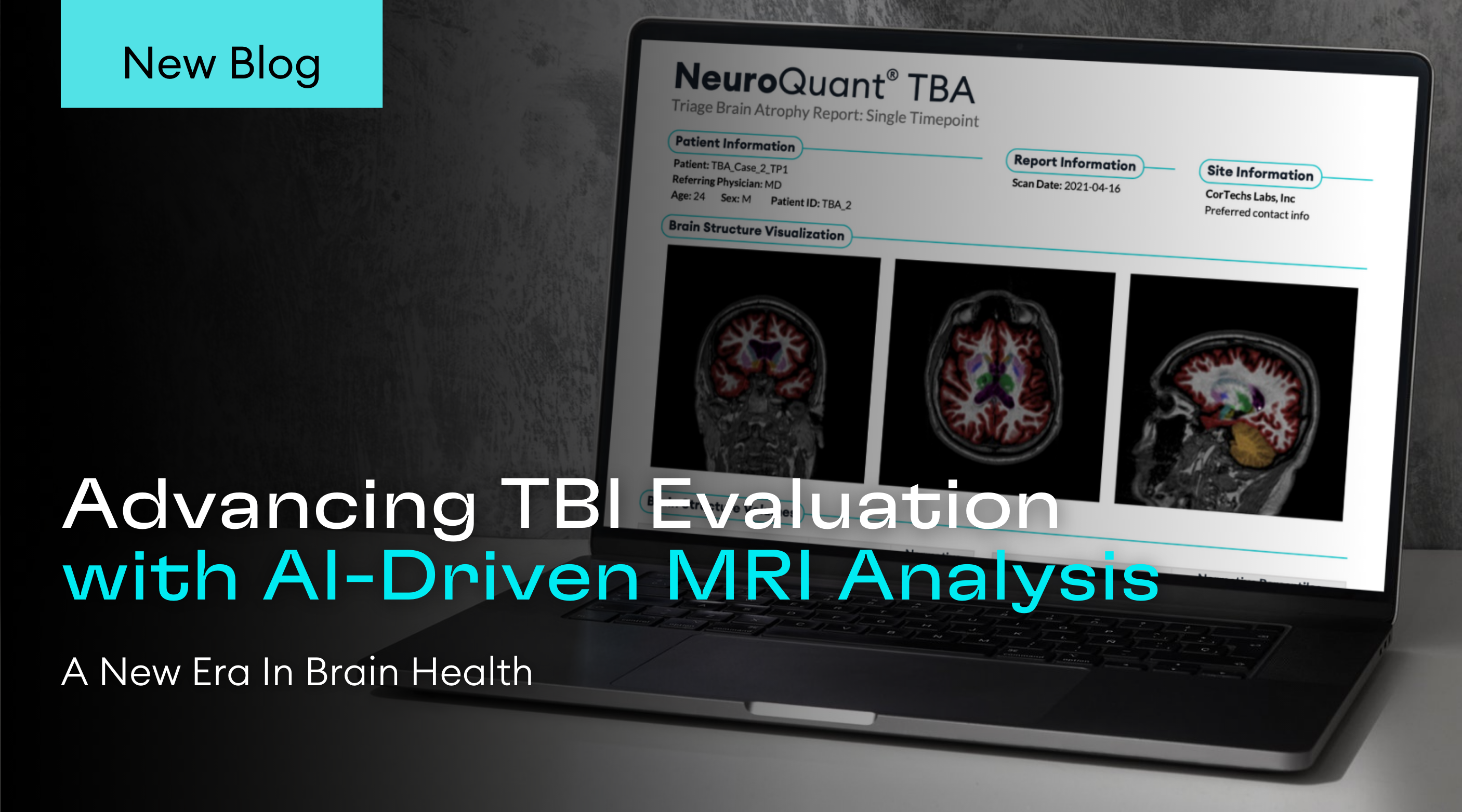
San Diego, CA – Cortechs.ai, a pioneer in AI-driven healthcare solutions, proudly announces a major update for its flagship product, NeuroQuant® v5.0, which is currently FDA 510(k) pending. This cutting-edge advancement marks a milestone in the field of neuroimaging AI, empowering healthcare professionals with unparalleled precision in diagnosing and monitoring neurological disorders.
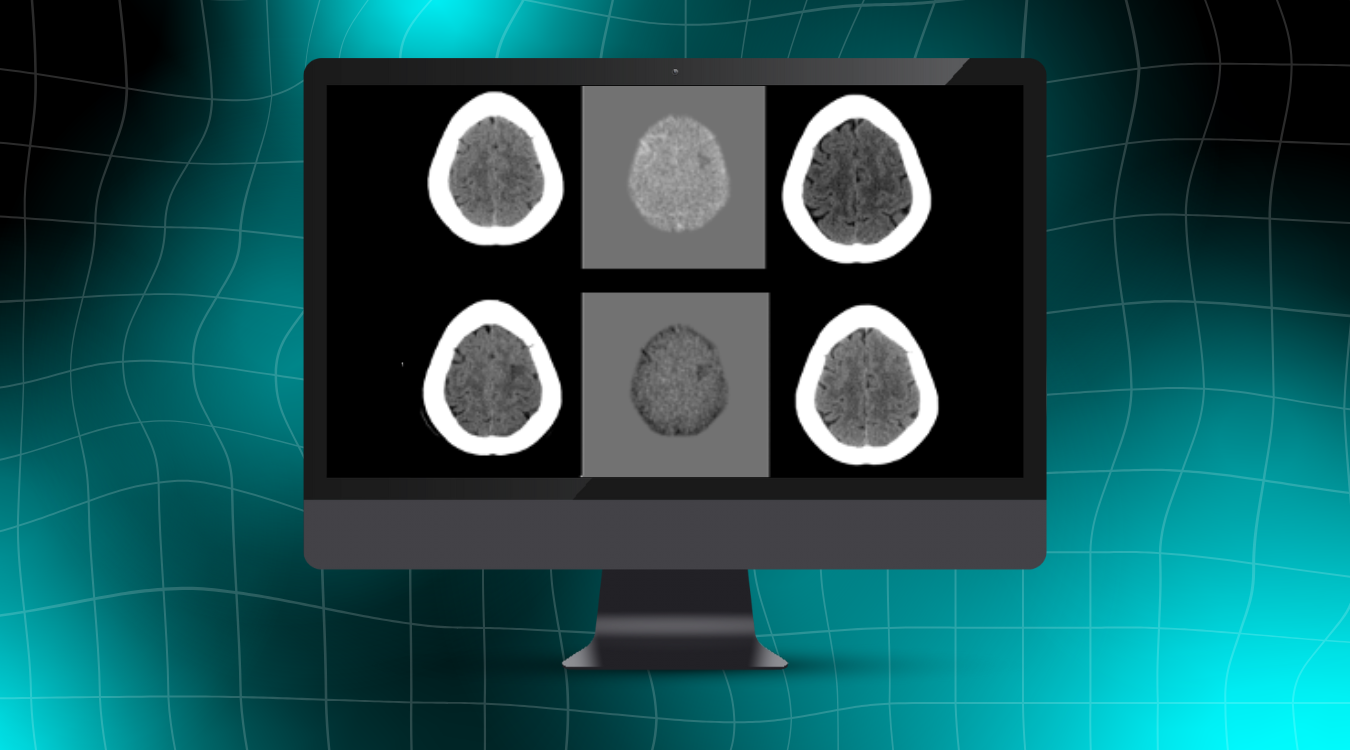
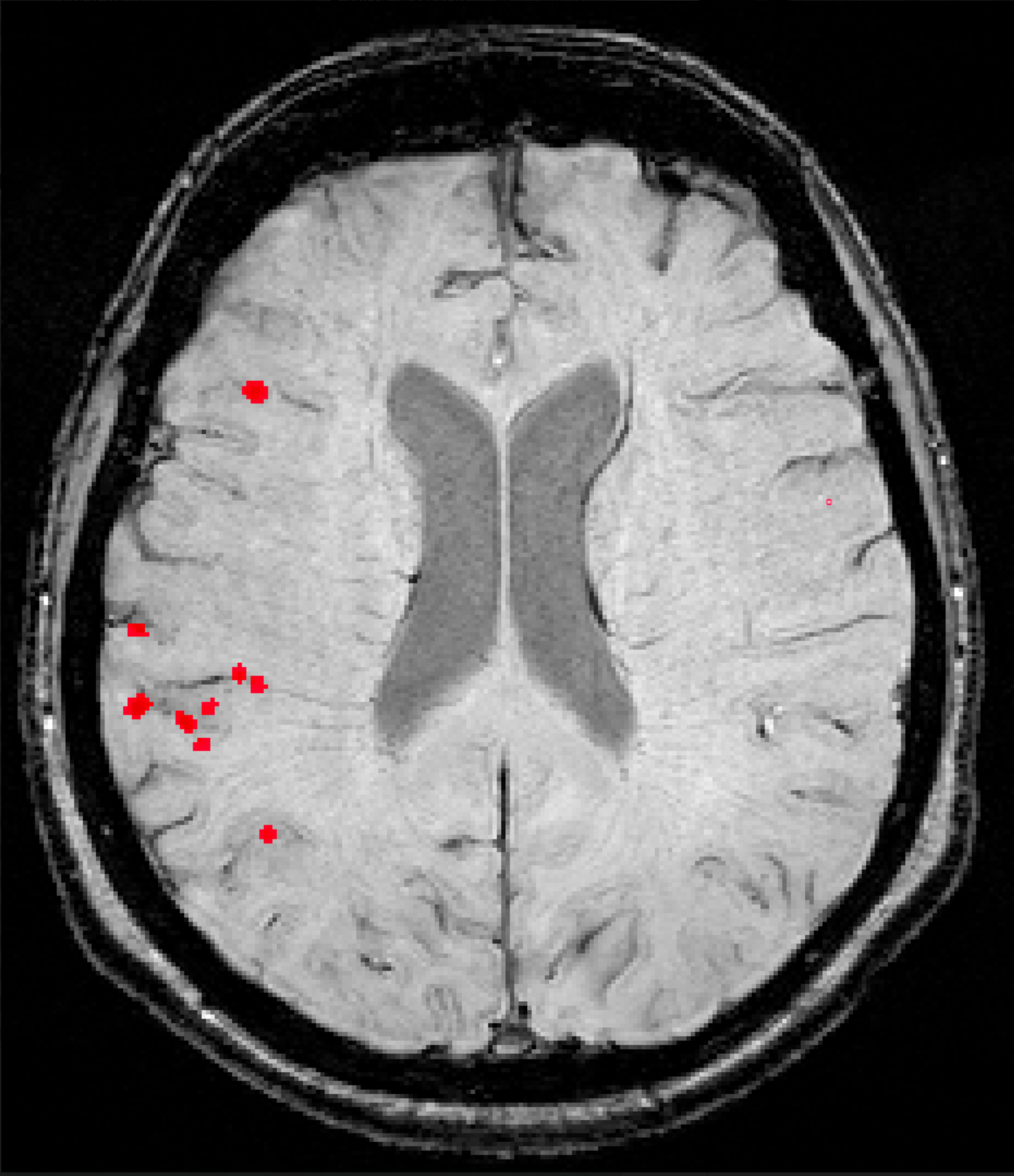
This latest update to NeuroQuant® will expand the use of the software to support identification and evaluation of cerebral microhemorrhages on susceptibility-sensitive MRI sequences – small focal brain lesions commonly found in patients with Traumatic Brain Injuries (TBI), Cerebral Amyloid Deposition Diseases including Cerebral Amyloid Angiopathy (CAA) and Alzheimer’s Disease (AD), as well as patients with Amyloid Related Imaging Abnormalities (ARIA).
“Our upcoming NeuroQuant® v5.0 application empowers healthcare professionals with precise data to inform diagnoses and monitor brain health. This innovation underscores our commitment to advancing neurological care for radiologists, neurologists, researchers, and patients alike.” says Brandon Steach, Head of Product at Cortechs.ai.
Nate White, Ph.D and Chief Technology Officer, says “Since the first commercial launch of NeuroQuant® in 2006, Cortechs continues to lead our industry, driving transformation through pioneering initiatives and fueling innovation in imaging-based AI technologies. The release of NeuroQuant® v5.0 strengthens our commitment to staying ahead of the curve and continuously pushing boundaries to provide AI-driven solutions to optimize patient care.”
About Cortechs.ai
Cortechs.ai is a leader in AI applications in radiology, using cutting-edge advances in medical imaging to revolutionize disease screening and early detection so patients can enjoy longer, healthier lives. The company develops and markets breakthrough medical device software that quantifies and tracks neurodegenerative diseases and assists in the detection of clinically significant cancer. Cortechs.ai’s industry-leading brain imaging analysis provides radiologists, neurologists, oncologists, and clinical researchers worldwide with a convenient and cost-effective means to quantify brain structures to help assess neurological conditions, such as Alzheimer’s disease, epilepsy, multiple sclerosis, brain trauma, and brain development abnormalities. The company has FDA-cleared products for use in the diagnosis and follow up of neurodegenerative and traumatic brain conditions, as well as prostate cancer. Please visit www.cortechs.ai for further information and follow us on Twitter, LinkedIn, and Facebook.
For more information on Cortechs.ai and the upcoming NeuroQuant® release, please contact:
___________
info@cortechs.ai
+1 858 459 9700