By
Cortechs.ai
2 mins
Gadolinium (Gd), a common MRI contrast agent, makes certain tissues appear brighter (or enhanced) as compared to surrounding tissues by causing surrounding water molecules to give off a stronger signal and provide better image quality in some areas (Malikova & Holesta, 2017).
Since Gd enhances visibility and definition of boundaries of lesions, a brain MRI with contrast is beneficial in indicating active disease and can help confirm a diagnosis of diseases, such as Multiple Sclerosis (MS) by indicating enhancing areas of uptake from lesions. Overtime, follow-up MRI scans with contrast can show new lesions and indicate regions that are continuing to display evidence of active disease.
Recently, concerns have been raised by physicians and researchers regarding the repeated use of gadolinium as an MRI contrast agent. These studies have found that minor traces of Gd contrast can accumulate in brain structures over time (Lohre et al., 2017), and the long-term effects of that accumulation are unknown (Tedeschi et. Al., 2017; Malikova & Holesta, 2017). In March 2017, the European Medicines Agency (EMA) recommended reducing to completely halting the use of certain Gd contrast agents (PRAC, 2017).
These recent recommended MRI protocol changes have created a gap for physicians in their evaluation of lesion appearance and progression.
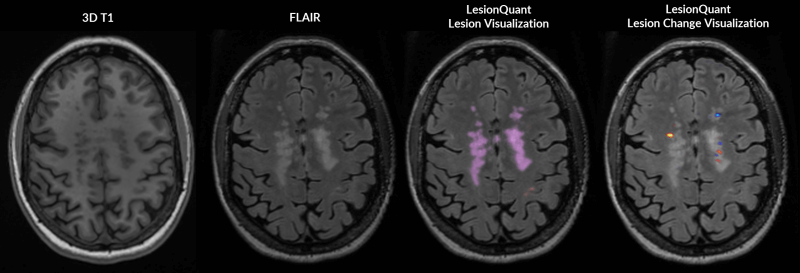
LesionQuant, a NeuroQuant product, can help bridge that gap. Through the combination of structural 3D T1 and FLAIR MRI, LesionQuant can aid physicians in monitoring lesion progression by quantifying FLAIR lesion count, volume and change. Using LesionQuant, neurologists and radiologists retain the ability to quickly and accurately identify and monitor new and enlarging lesions, all without the need or added risk of Gd.
LesionQuant takes these measurements a step further by providing a DICOM color overlay that highlights areas of increasing or decreasing FLAIR hyperintensity, which can indicate active or growing lesions, or lesions that are in the process of resolving.
 [divider style= double” padding_top=”5″ padding_bottom=”5″][/divider]
[divider style= double” padding_top=”5″ padding_bottom=”5″][/divider]
References:
Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency. PRAC concludes assessment of gadolinium agents used in body scans and recommends regulatory actions, including suspension for some marketing authorisations. (2017, March 10). Retrieved April 03, 2017, from http://www.ema.europa.eu/ema/index.jsp?curl=pages%2Fnews_and_events%2Fnews%2F2017%2F03%2Fnews_detail_002708.jsp&mid=WC0b01ac058004d5c1
Lohrke J, Frisk AL, Frenzel T, Schöckel L, Rosenbruch M, Jost G, Lenhard DC, Sieber MA, Nischwitz V, Küppers A, Pietsch H. Histology and Gadolinium Distribution in the Rodent Brain After the Administration of Cumulative High Doses of Linear and Macrocyclic Gadolinium-Based Contrast Agents. Invest Radiol. 2017 Mar 20. doi: 10.1097/RLI.0000000000000344. [Epub ahead of print] PubMed PMID: 28323657.
Malikova H, Holesta M. Gadolinium contrast agents – are they really safe? J Vasc Access. 2017 Mar 21:0. doi: 10.5301/jva.5000713. [Epub ahead of print] PubMed PMID: 28362042.
Robert J. McDonald, Jennifer S. McDonald, David F. Kallmes, Mark E. Jentoft, David L. Murray, Kent R. Thielen, Eric E. Williamson, and Laurence J. Eckel. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015 275:3, 772-782
Tedeschi E, Caranci F, Giordano F, Angelini V, Cocozza S, Brunetti A. Gadolinium retention in the body: what we know and what we can do. Radiol Med. 2017 Mar 30. doi: 10.1007/s11547-017-0757-3. [Epub ahead of print] Review. PubMed PMID: 28361260.
Share