By
Cortechs.ai
2 mins
By: Marilyn Maes MS, RA, RT (R), (MR)
Overview:
Physicians use several evaluation tools to make accurate assessments of memory loss in patients, one of them is a magnetic resonance imaging (MRI) scan of the patient’s brain. A NeuroQuant analysis can be used with the routine MRI brain scan to provide additional insight.
NeuroQuant, the first FDA 510(k) cleared medical software for quantitative analysis of brain MRI, provides brain structure volumetrics to assist physicians in fast and accurate diagnoses of neurodegenerative diseases.
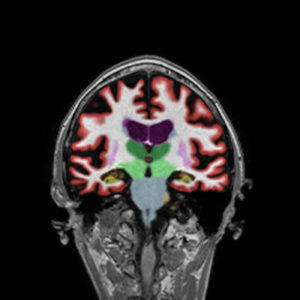
When assessing dementia, NeuroQuant uses images from the MRI scan to measure the volume of brain structures relevant in memory loss pathologies. The software allows physicians to identify particular areas of concern, such as the hippocampus, entorhinal cortex, and inferior lateral ventricles.
NeuroQuant provides reference percentiles for structures using an extensive covariate modulated normative database with ages from 3 – 100 years of age, both genders and ethnicities from around the globe. The structure volumes are compared to healthy subjects of the same age and sex, then normalized by intracranial volume (ICV) to provide a normal percentile between 1 and 99. Structure volumes falling outside of the 5th-95th percentile are typically of diagnostic importance.
AD is one of the most notorious and the most common forms of dementia, accounting for 60% to 90% of the dementing disorders with progressive memory loss often with personality changes and impaired cognition.
AD brains demonstrate specific patterns of atrophy predominantly in regions mediating cognitive functions such as the temporal lobes. In most AD pathologies the atrophy distribution follows this pattern, hippocampus initially, with the entorhinal cortex first. Then, hippocampal and amygdala atrophy and global atrophy. Late stages are generally bilateral, symmetric, and diffuse. The disease advancement is from the entorhinal cortex to the hippocampus to the neo-cortex as a rule.
NeuroQuant was used part of an AD work up on a 79-year-old, female patient and demonstrates an early AD pattern of atrophy.
Alzheimer’s Disease in NeuroQuant
Results:
Head MRI: Revealed cortical atrophy and periventricular white matter changes. No tumor, hemorrhage, subdural hematoma, or large cerebral infarct.
PET: Neuronal injury (FDG PETQuant, structural MRI)
Neuropsychologic: Confirmed mild dementia, with deficits in memory, language, visuospatial skills, and frontal/executive function.
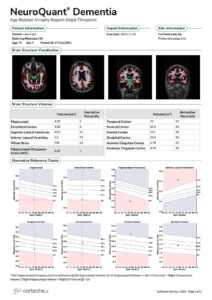
NeuroQuant: Analysis demonstrates statistically significant hippocampal atrophy in the 1st percentile and statistically significant compensatory inferior lateral ventricle enlargement in the 99th percentile. There is also statistically significant reduction of the HOC in the 1st percentile, and borderline low entorhinal cortex volume in the 6th percentile. Suggestive of early dementia due to Alzheimer’s disease.
References:
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl 1996; 165: 3–12.
- Jack CR jr., Petersen RC, Xu YC et al. Medial tem- poral atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology 1997; 49: 786 – 94.
- Scheltens P, Leys D, Barkhof F et al. Atrophy of medial temporal lobes on MRI in «probable» Alz- heimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 1992; 55: 967–72.
- Jobst KA, Smith AD, Barker CS et al. Association of atrophy of the medial temporal lobe with reduced blood flow in the posterior parietotemporal cortex in patients with a clinical and pathological diagno- sis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1992; 55: 190–4.
- Oksengaard AR, Haakonsen M, Dullerud R et al. Accuracy of CT scan measurements of the medial temporal lobe in routine dementia diagnostics. Int J Geriatr Psychiatry 2003; 18: 308–12.
- Brewer JB, Magda S, Airriess C et al. Fully-auto- mated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease. AJNR Am J Neuroradiol 2009; 30: 578–80.
- McEvoy LK, Fennema-Notestine C, Roddey JC et al. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impair- ment. Radiology 2009; 251: 195–205.
- Brewer JB. Fully-automated volumetric MRI with normative ranges: translation to clinical practice. Behav Neurol 2009; 21: 21–8.
- Wahlund LO, Almkvist O, Blennow K et al. Evi- dence-based evaluation of magnetic resonance imaging as a diagnostic tool in dementia workup. Top Magn Reson Imaging 2005; 16: 427–37.
- Bottino CM, Castro CC, Gomes RL et al. Volume- tric MRI measurements can differentiate Alzhei- mer’s disease, mild cognitive impairment, and normal aging. Int Psychogeriatr 2002; 14: 59–72.
- Pennanen C, Kivipelto M, Tuomainen S et al. Hip- pocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging 2004; 25: 303–10.
- Andersson C, Lindau M, Almkvist O et al. Identify- ing patients at high and low risk of cognitive decline using Rey Auditory Verbal Learning Test among middle-aged memory clinic outpatients. Dement Geriatr Cogn Disord 2006; 21: 251–9.
- Almkvist O, Tallberg IM. Cognitive decline from estimated premorbid status predicts neurodege- neration in Alzheimer’s disease. Neuropsychology 2009; 23: 117–24.
- Mayeux R, Saunders AM, Shea S et al. Utility of the apolipoprotein E genotype in the diagnosis of Alz- heimer’s disease. N Engl J Med 1998; 338: 506–11.
- Ince PG, McArthur FK, Bjertness E et al. Neuro- pathological diagnoses in elderly patients in Oslo: Alzheimer’s disease, Lewy body disease, vascular lesions. Dementia 1995; 6: 162–8.
- Merdes AR, Hansen LA, Jeste DV et al. Influence of Alzheimer pathology on clinical diagnostic accur- acy in dementia with Lewy bodies. Neurology 2003; 60: 1586–90.
- Galasko D, Hansen LA, Katzman R et al. Clinical- neuropathological correlations in Alzheimer’s disease and related dementias. Arch Neurol 1994; 51: 888–95.
Share


