By George Mastorakos
Brain tumors are a serious and often life-threatening condition that can affect anyone at any age. According to the American Brain Tumor Association[1], nearly 80,000 new cases of primary brain tumors are diagnosed. Among these diagnoses, gliomas are the most common type of primary malignant brain tumor. Gliomas originate from glial cells, which support and protect neurons in the brain and spinal cord. Unfortunately, gliomas can be aggressive and difficult to treat and/or surgically remove due to their location in the brain. They account for approximately 80% of all malignant brain tumors and are associated with high morbidity and mortality rates. The severity of gliomas can vary widely depending on the subtype, stage of the disease, and individual patient factors[2]. Patients with gliomas may experience a range of symptoms, including headaches, seizures, cognitive impairments, and motor dysfunction, which can significantly impact their quality of life.
Accurate longitudinal follow-up of patients affected by gliomas require precise measurement of tumor volume and location. Currently, the predominant standard method of measuring glioma size is painstaking manual 2D measurements, which are highly crude, variable, and time-consuming. These measurements become exponentially critical in the context of clinical trials. Thus, there lies a growing need for more sophisticated, precise, automated techniques that can deliver improved measures near-instantaneously.
Enter NeuroQuant® Brain Tumor[3], an FDA 510k-cleared, AI solution that provides automated, image-based 3D volumetric analyses of previously diagnosed brain tumors straight to a physician’s fingertips in PACS. Below, we review the value additions this app can make to neurological clinical workflows in the context of assessing a patient affected by glioma.
Case Study 1: High-Grade Glioma
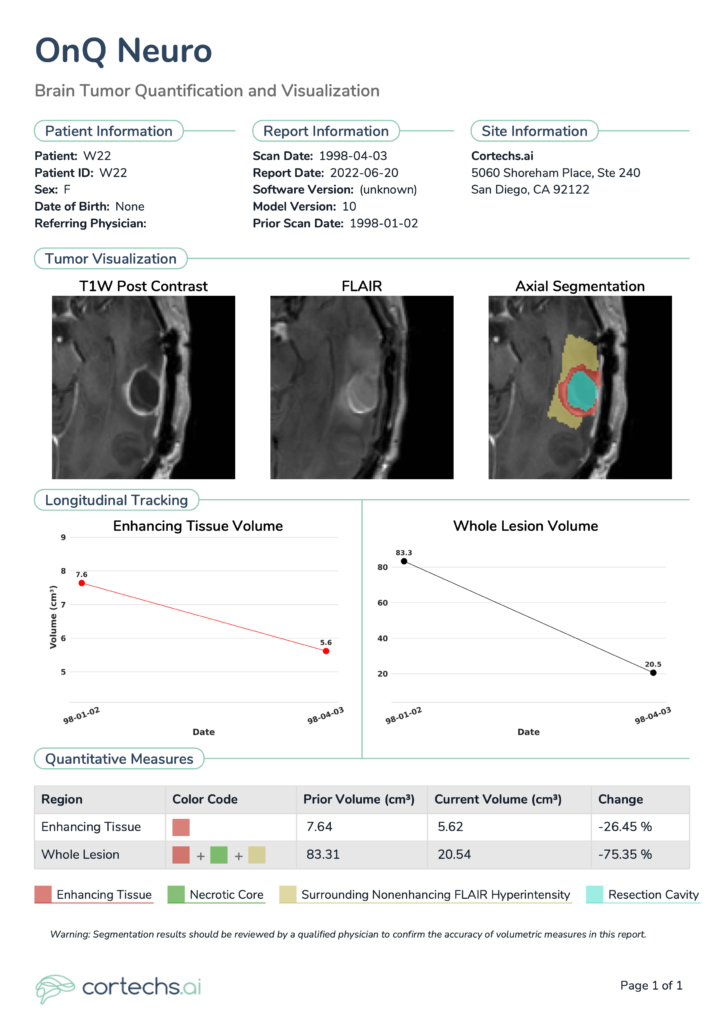
This case highlights a female patient, aged in her 50s, who had been diagnosed with a high-grade glioma. The patient was considered a candidate for surgery and underwent neurosurgical resection. Two time points are analyzed in this case, the first time point demonstrating the preoperative MRI scan (Figure 1) and the second demonstrating a follow-up scan approximately 3 months after resection (Figure 2).
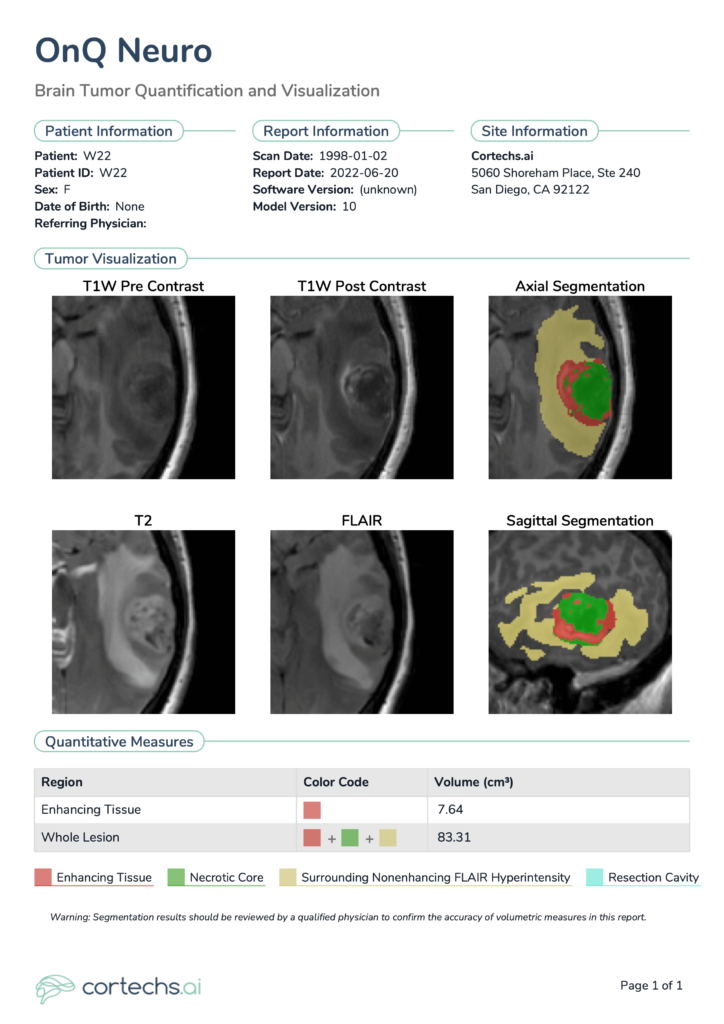
Shown at the top of the single time point NeuroQuant® Brain Tumor Report are patient details and relevant information, including software version, scan date and imaging location. Tumor visualizations include the color-coded segmentation overlay, configurable representative segmentation image in the axial and sagittal planes, as well as multimodal images: T1 pre-contrast, T1 post-contrast, T2, FLAIR. Quantitative measures are shown toward the bottom. For follow-up, longitudinal timepoint reports, additional plots are included to provide a snapshot of all previous timepoint volume data points for a given patient.
NeuroQuant® Brain Tumor provides volume of different regions of interest of the tumor, lists them in tabular format, and provides a clear snapshot of any previous volumes (Figure 2). In this case, NeuroQuant® Brain Tumor identified an enhancing tissue volume of 5.62 cm3, a 26.45% decrease relative to the prior report (7.64 cm3). Whole lesion volume measured 20.54 cm3, a 75.35% decrease relative to the prior report (83.31 cm3).
Case Study 2: Low-Grade Glioma
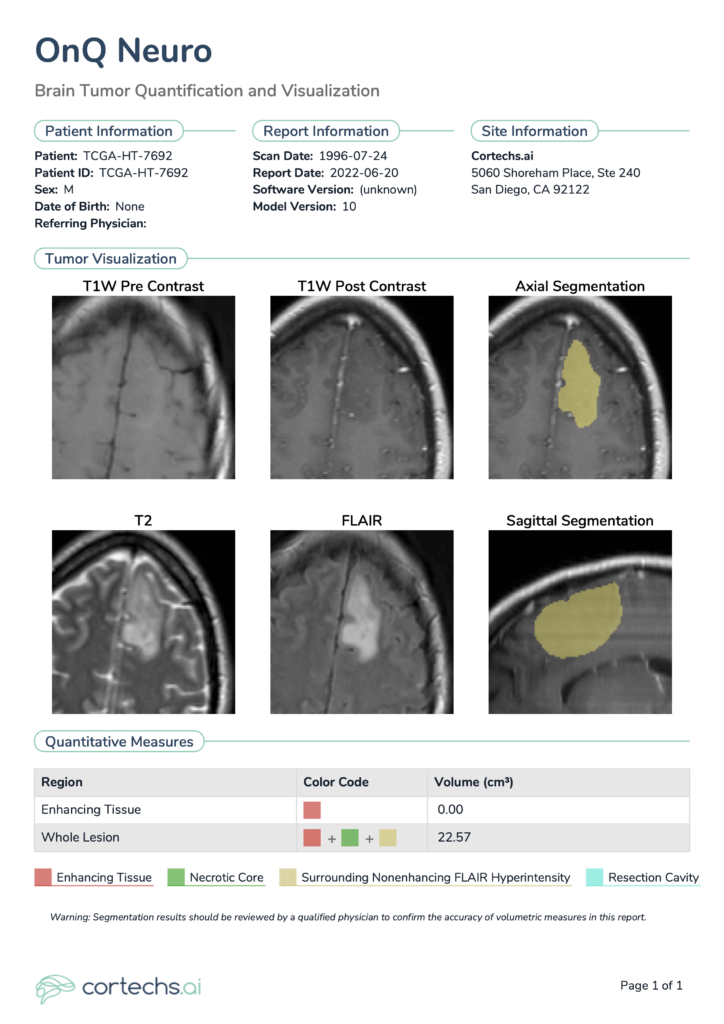
NeuroQuant® Brain Tumor also can segment not only preoperative and postoperative high-grade gliomas, but low-grade gliomas as well. Figure 3 shows the report pertaining to the case of a male patient, aged in his 40s. As is typical for well-differentiated low-grade tumors4, no enhancing tissue was identified in this case. However, whole lesion volume measured 22.57 cm3 and consisted only of the Surrounding Nonenhancing FLAIR Hyperintensity region of interest in this particular case.
During the treatment course and longitudinal assessment of brain tumor patients, neuro-oncologists and care teams deserve convenient, consistent tools for tracking patient status. NeuroQuant® Brain Tumor provides objective tumor volumetrics for both high-grade and low-grade gliomas, as well as longitudinal volumetric assessments pre- and post-operatively.
References
- Faris, J. Glioblastoma (GBM). American Brain Tumor Association https://www.abta.org/tumor_types/glioblastoma-gbm/ (2022).
- Grochans, S. et al. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 14, 2412 (2022).
- OnQ Neuro. Cortechs.ai https://www.cortechs.ai/onq-neuro/ (2022).
- Youssef, G. & Miller, J. J. Lower Grade Gliomas. Curr. Neurol. Neurosci. Rep. 20, 1–9 (2020).