Sustaining any type of brain trauma can be very serious and there can be potential complications. Brain trauma is a risk factor for a variety of other brain-related problems, including chronic headaches, insomnia, and undiagnosed aneurysms. Magnetic resonance imaging (MRI) is one of the most sensitive diagnostic imaging tests available. This technology is useful for evaluating a wide range of brain disorders including traumatic injury, tumors, stroke, and aneurysms.
A special MRI scanning technique called diffusion tensor imaging (DTI) can be used to help diagnose aneurysms or other brain problems that may not appear on a routine MRI scan. A thorough assessment will include a NeuroQuant® analysis, which is an advanced 3D volumetric post-processing software that automatically identifies and segments brain structures, measures their volumes, and compares the information to a normative database adjusted for age, gender, and cranial volume.
Emerging imaging technologies like MR spectroscopy (MRS) in TBI is being used to investigate changes in brain metabolites, such as reduced N-acetylaspartate (NAA) levels, which may demonstrate a loss of neuronal and axonal integrity.
Let’s take a closer look at this case study.
- Patient: Mya Pain
- DOB: 12/28/1972
- History: Brain trauma 10/10/2022
- Date of Exam: 3/24/23
Multi-modal MRI technique:
- MRI Brain: MRI examination of the brain was performed on a Siemens Trio 3.0 Tesla scanner utilizing sagittal 3D 1.1 mm 3D ADNI, T2/FLAIR, with multiplanar reformation, axial fat sat T2, and axial 3D SWI.
- NeuroQuant®: The 3D ADNI scan was analyzed by NeuroQuant® (Cortech’s Labs), a 3D morphometric technique utilizing an anatomic atlas based upon >3000 age (between 3 and 100 years) and gender-matched controls yielding percentile volume scores.
- DTI Brain: Diffusion-tensor imaging (DTI) was performed on a Siemens Trio 3.0 Tesla scanner utilizing 30 directions with 5 mm sections, 1.5 mm gap, 230 mm field of view, TE/TR 106/3000 ms, two averages, AP phase, 128×128 matrix, 1.8 x 1.8 x 5.0 mm voxel size with 1/5mm slice gap, GRAPPA acceleration factor 2, 30 reference lines, b values of 0 s/mm2, and 2000 s/mm2, bw = 1396 Hz/Px and EPI factor of 128.
- 2D-CSI Spectroscopy (Chemical Shift Imaging): 2D-CSI spectroscopy of the centrum semiovale was performed on a Siemens Trio 3.0 Tesla scanner utilizing 16 voxels placed within the centrum semiovale centrally, oriented parallel to the Anterior-posterior commissure line, having voxel (ROI) dimensions of 15 mm3. Sequence parameters were TE/TR 135 ms/1700 ms, with three averages; BW = 1200, vector size = 1024; width = 50; flip angle = 90 degrees; weak water suppression at 50 Hz, without spectral suppression. ROIs within the centrum semiovale were analyzed in eight locations; the outer row of 4 ROIs centered within the midportion of each centrum semiovale were analyzed; the center two rows of ROIs positioned within the gray matter were not analyzed, yielding four ROIs on the right and four on the left, numbered front-to-back: Cho/Naa, Cr/Naa and Cho/Cr 1-4 and 5-8.
Findings:
- MRI Brain: Few abnormal foci of increased T2/FLAIR signal detected within the white matter. Orbits, sella and parasellar regions, pineal region, cerebellopontine angles and craniovertebral junction are unremarkable. No cyst, mass, or congenital anomaly detected. No abnormal fluid collection: no intracranial bleed of any age identified with particular attention to the SWI sequence. Sinuses are clear; no mastoid fluid.
- NeuroQuant® TBA: Hippocampal volumes are at the 96th and 51st percentiles on the left and right respectively. Ventricular volume is at the 8th percentile. Global cortical gray matter volume is at the 58th percentile. Deep gray nuclei: left putamen 2nd percentile, right 7th percentile, left thalamus 1st percentile, right 57th percentile, left caudate 1st percentile, right 14th. Posterior cingulate gyrus left 1st percentile, right 3rd percentile.
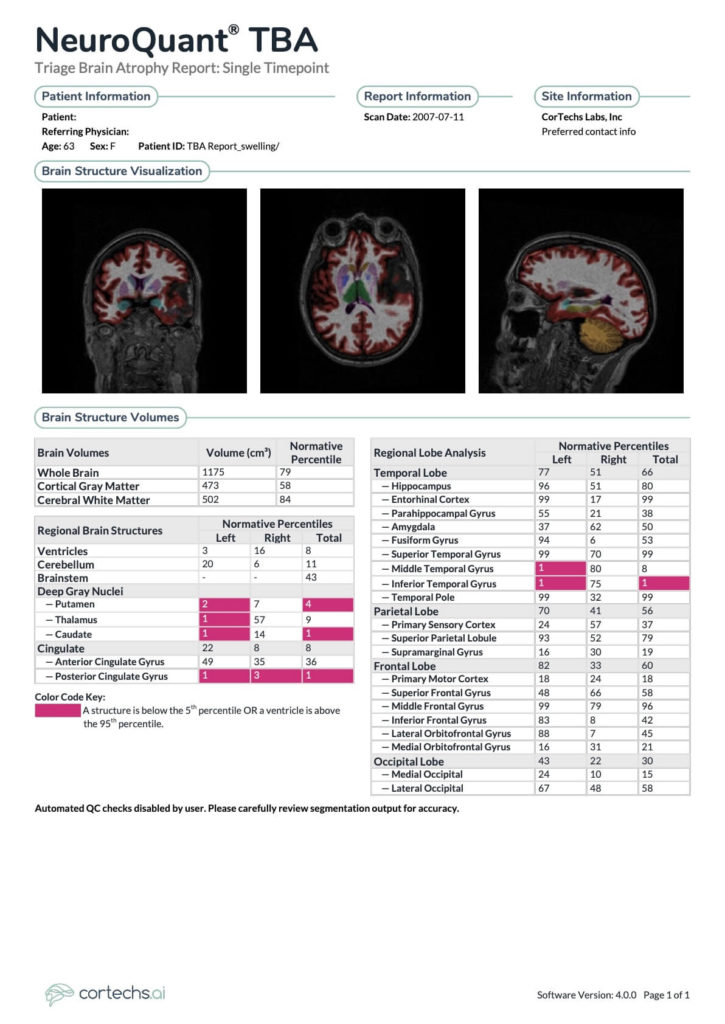
The NeuroQuant TBA report - DTI Brain: Statistically significant FA reduction is present within the genu of the corpus callosum.
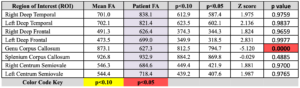
DTI table - 2D-CSI Spectroscopy (Chemical Shift Imaging): Statistically significant major metabolite ratio derangement is present.
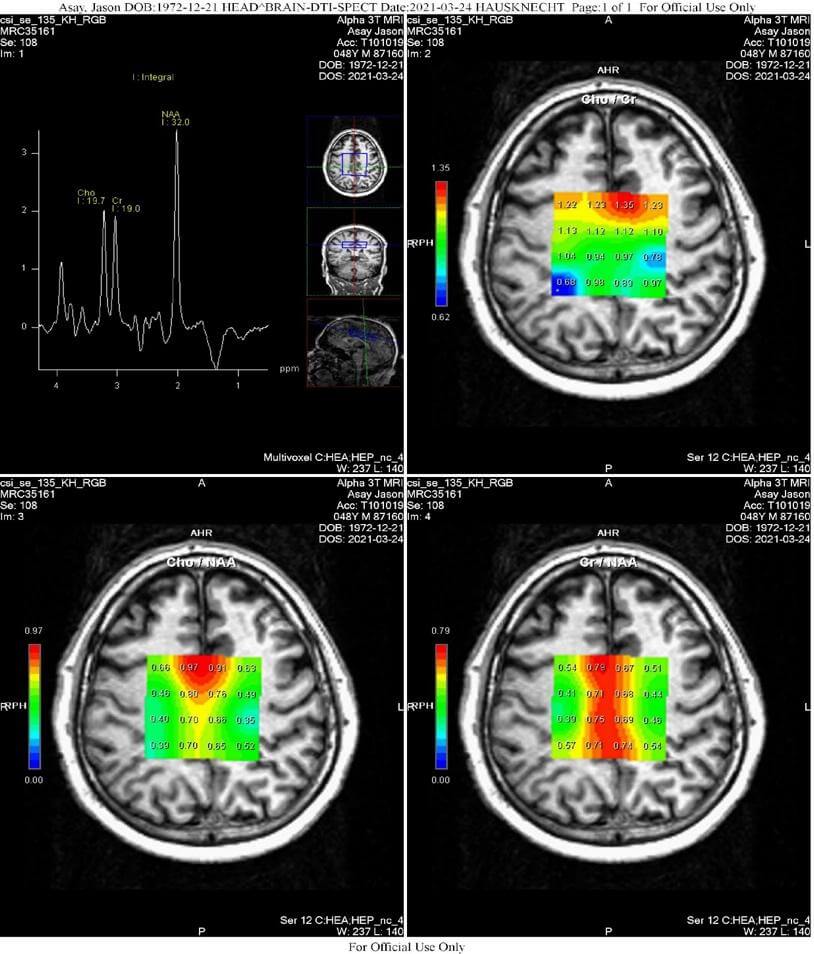
2D-CSI Spectroscopy (Chemical Shift Imaging)
Impressions:
- Statistically significant FA reduction on DTI analysis within the genu of the corpus callosum, most compatible with traumatic brain injury.
- Statistically significant derangement of major metabolite ratios within the centrum semiovale most compatible with traumatic brain injury.
- Clinical correlation is needed; follow-up multi-modal MRI examination including NeuroQuant® and spectroscopy is recommended at clinically appropriate time.
References:
- Alosco, M, et al. (2017). Magnetic Resonance Spectroscopy as a Biomarker for Chronic Traumatic Encephalopathy. Seminars in Neurology. 37. 503-509.
- Bigler ED. 2013. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci, 7:395.
- Brown M, et al. (2018). Magnetic resonance spectroscopy abnormalities in traumatic brain injury: A meta-analysis. J Neuroradiol, 45(2), 123-129.
- Farb RI, et al. (2003). Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology, 60(9), 1418-24.
- Govindarajan KA, et al. (2016). Cortical Thickness in Mild Traumatic Brain Injury. J Neurotrauma, 33(20), 1809-1817.
- Huang, LA, et al. (2014). Study of white matter at the centrum semiovale level with magnetic resonance spectroscopy and diffusion tensor imaging in cerebral small vessel disease. Genet Mol.Res, 13(2): 2683-2690.
- Matis G, et al. (2012). Traumatic Brain Injuries and Diffusion Tensor Imaging – A Review. Recent Patents on Med Imag. 2. 36-50.
- Ross D, Graham T, Ochs A. (2012). Review of the evidence supporting the medical and legal use of NeuroQuant® in patients with traumatic brain injury. Psychol Inj and Law, 6(1), 75-80.
- Ross DE, et al. (2012). Progressive brain atrophy in patients with chronic neuropsychiatric symptoms after mild traumatic brain injury: a preliminary study. Brain Inj, 26(12), 1500-9.
- Vagnozzi R, et al. (2010). Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain, 133 (11), 3232–3242.
- Wilde EA, et al. (2018). Brain morphometric techniques applied to the study of traumatic brain injury. In: Spalletta G, Piras F, Gili T. (eds) Brain Morphometry. Neuromethods, vol. 136. Humana Press, New York, NY, 469-530.
- Tong K, Holshouser B, Shutter L, Chiou P, Herigauk G, Haacke E. High resolution susceptibility weighted imaging (SWI) improves detection of hemorrhagic lesions in adults with traumatic brain injury: correlation with severity of injury and outcome. Proceedings of ISMRM 10th Scientific Meeting. Berkeley: International Society for Magnetic Resonance in Medicine2002 :46 Google Scholar
- Sinson G, Bagley LJ, Cecil KM, et al. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: correlation with clinical outcome after traumatic brain injury. AJNR Am J Neuroradiol 2001;22:143–151 Abstract/FREE Full TextGoogle Scholar
- Brooks WM, Friedman SD, Gasparovic C. Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil 2001;16:149–164 PubMedGoogle Scholar
- Ashwal S, Holshouser BA, Shu SK, et al. Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatr Neurol 2000;23:114–125 CrossRefPubMedGoogle Scholar
- Brooks WM, Stidley CA, Petropoulos H, et al. Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J Neurotrauma 2000;17:629–640 CrossRefPubMedGoogle Scholar