Automated detection and quantification of ARIA-H and ARIA-E
Our AI software enables consistent, longitudinal tracking of patients receiving anti-amyloid therapies. Offering precise therapeutic surveillance for anti-amyloid medications, it improves patient monitoring and outcomes.
AI-Assisted Monitoring of Anti-Amyloid Immunotherapies for Alzheimer's Disease.
Comprehensive FLAIR lesion segmentation and identification – within minutes. With capabilities including precise therapeutic surveillance (including detection and tracking of lesion count, max diameter, and volume) for patients on anti-amyloid medications, NeuroQuant ARIA is designed to improve patient monitoring and outcomes.
Benefits
ARIA-E and ARIA-H
Precise monitoring of ARIA-H and ARIA-E lesion counts
OEM protocol standardization
To ensure reproducible outcomes and accuracy over time
1200 clinical sites
Extensive network of locations supports convenient patient access
Quantitative lesion detection
Use T2*GRE and FLAIR images to extract lesion quantitative measures
Longitudinal disease tracking
Monitor lesion counts and maximum diameters over time, with comparisons to baseline and prior timepoints
NeuroQuant® ARIA reports
Reports that assist in clinical decision-making by better characterizing ARIA, quantitating its occurrence, and assessing rate of change over time.
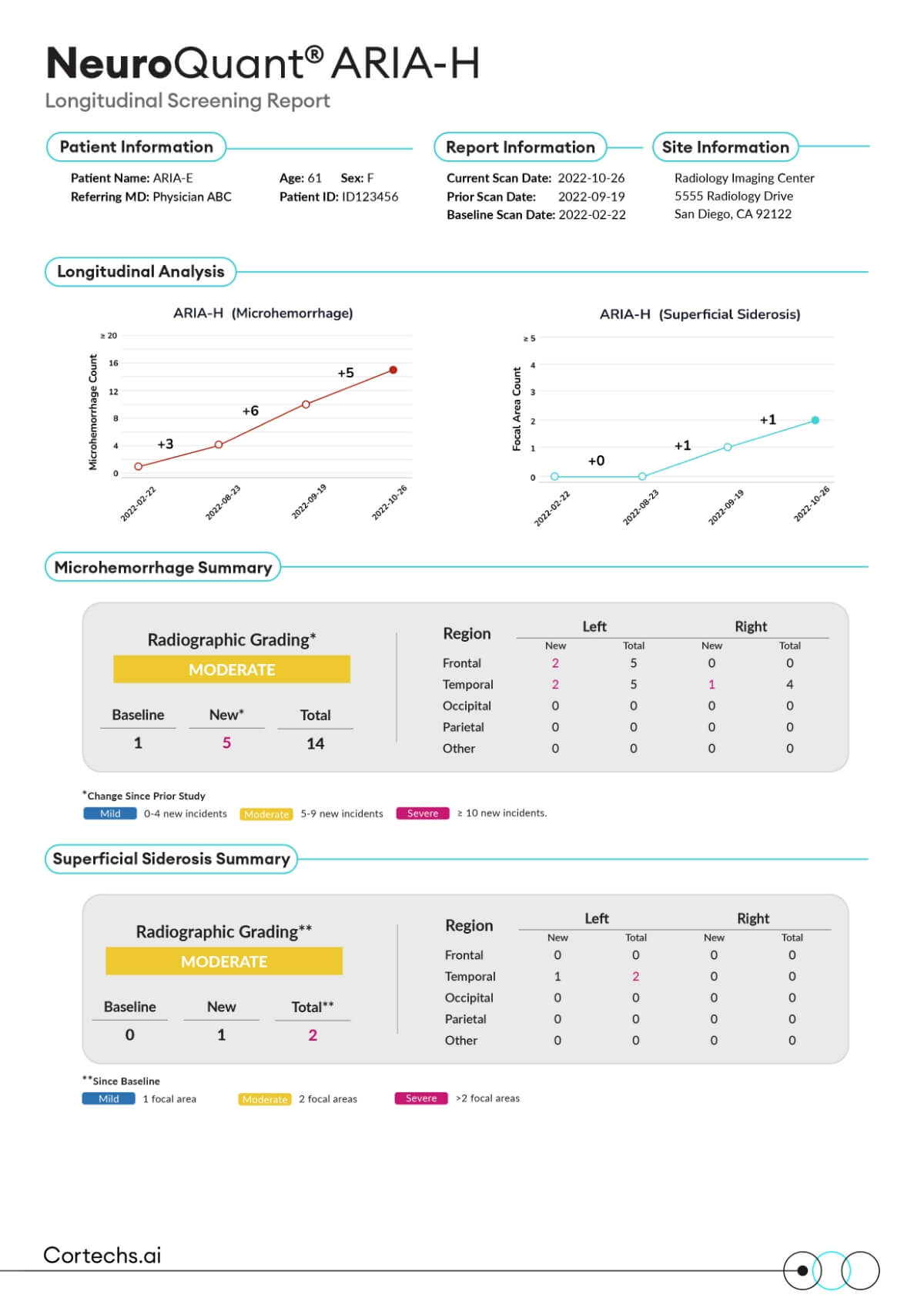
NeuroQuant® ARIA-H report provides automated microhemorrhage count and superficial siderosis area detection for patients receiving anti-amyloid therapies.
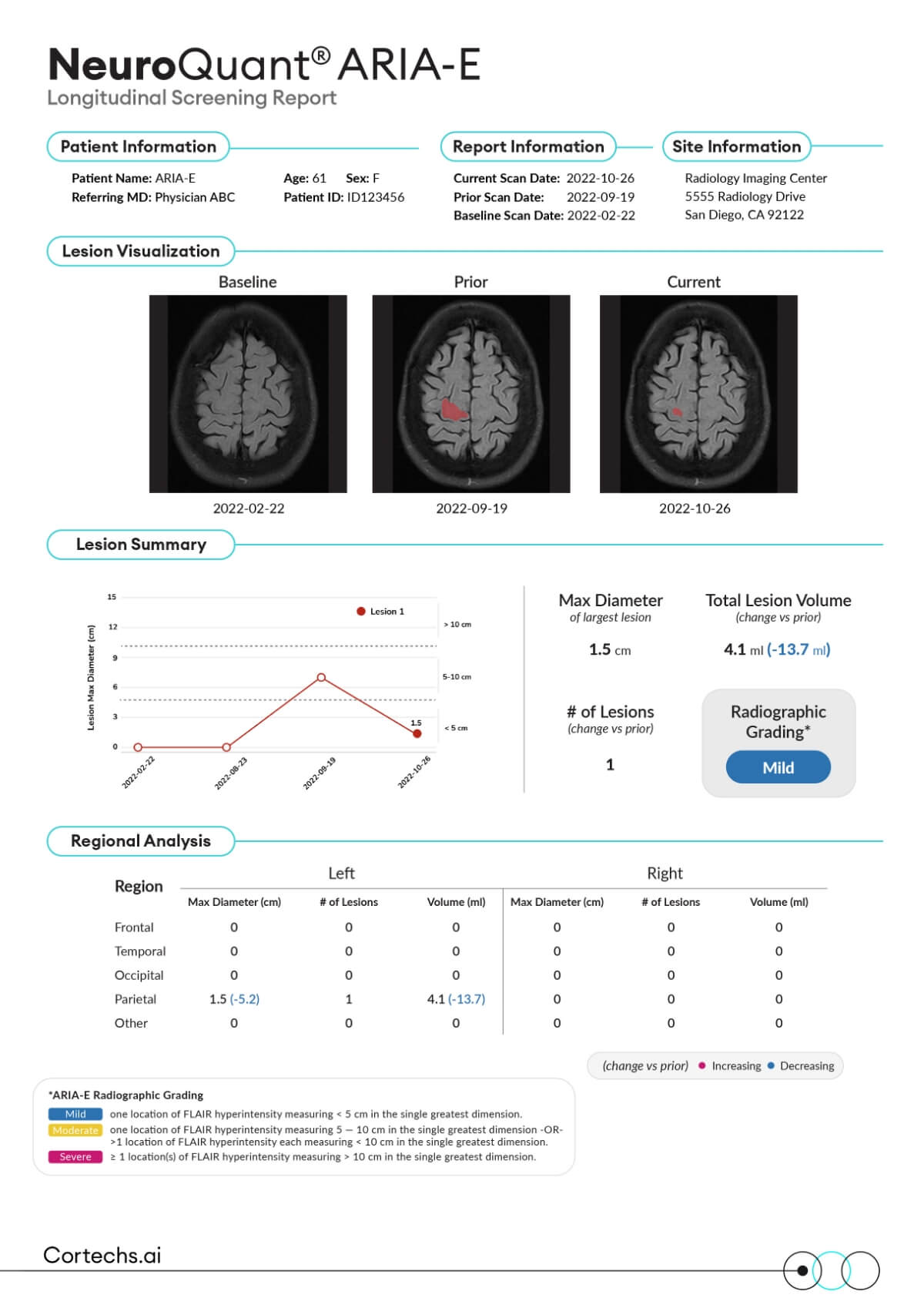
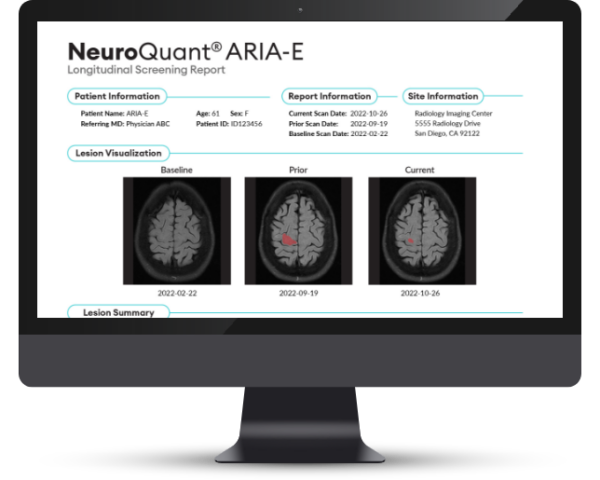
NeuroQuant® ARIA-E Report provides detection and tracking of lesion count, max diameter, and volume for patients receiving anti-amyloid therapies.


