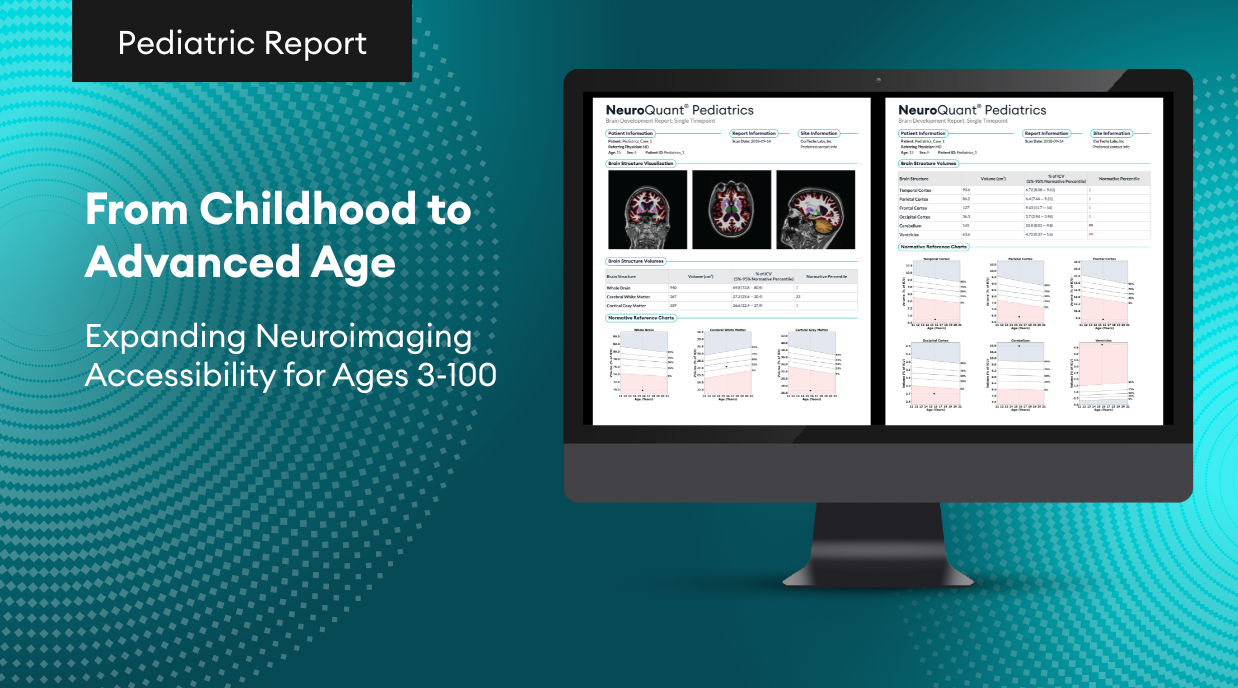
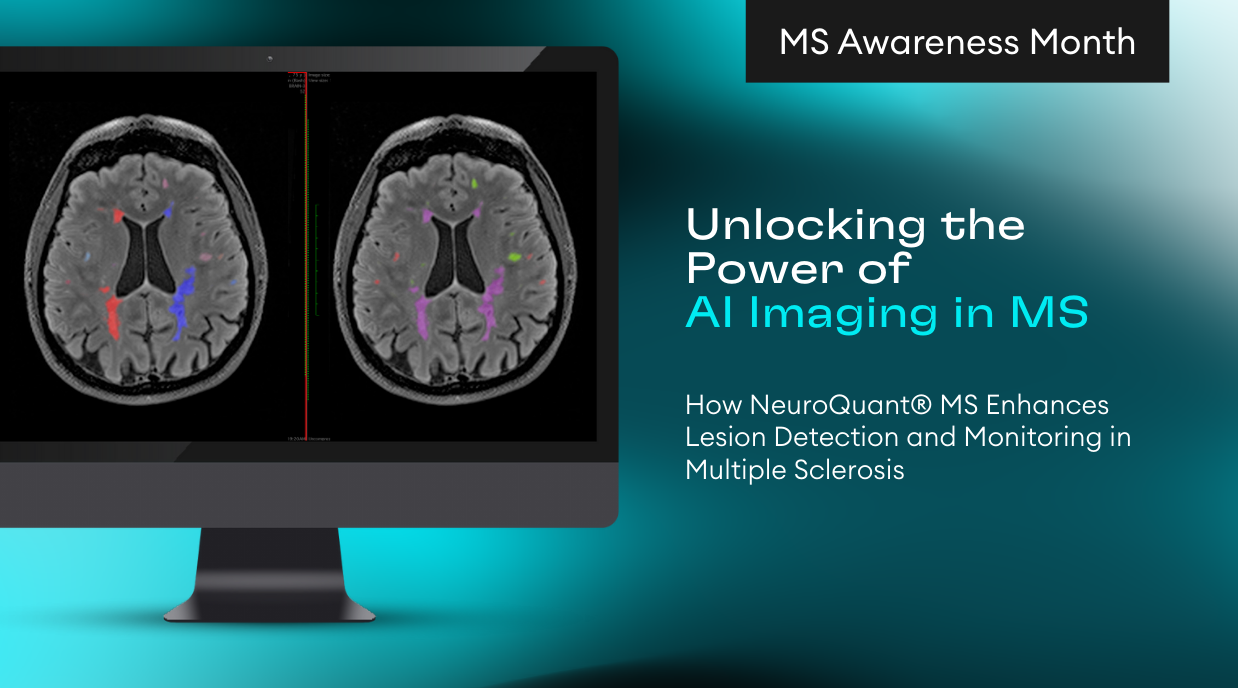
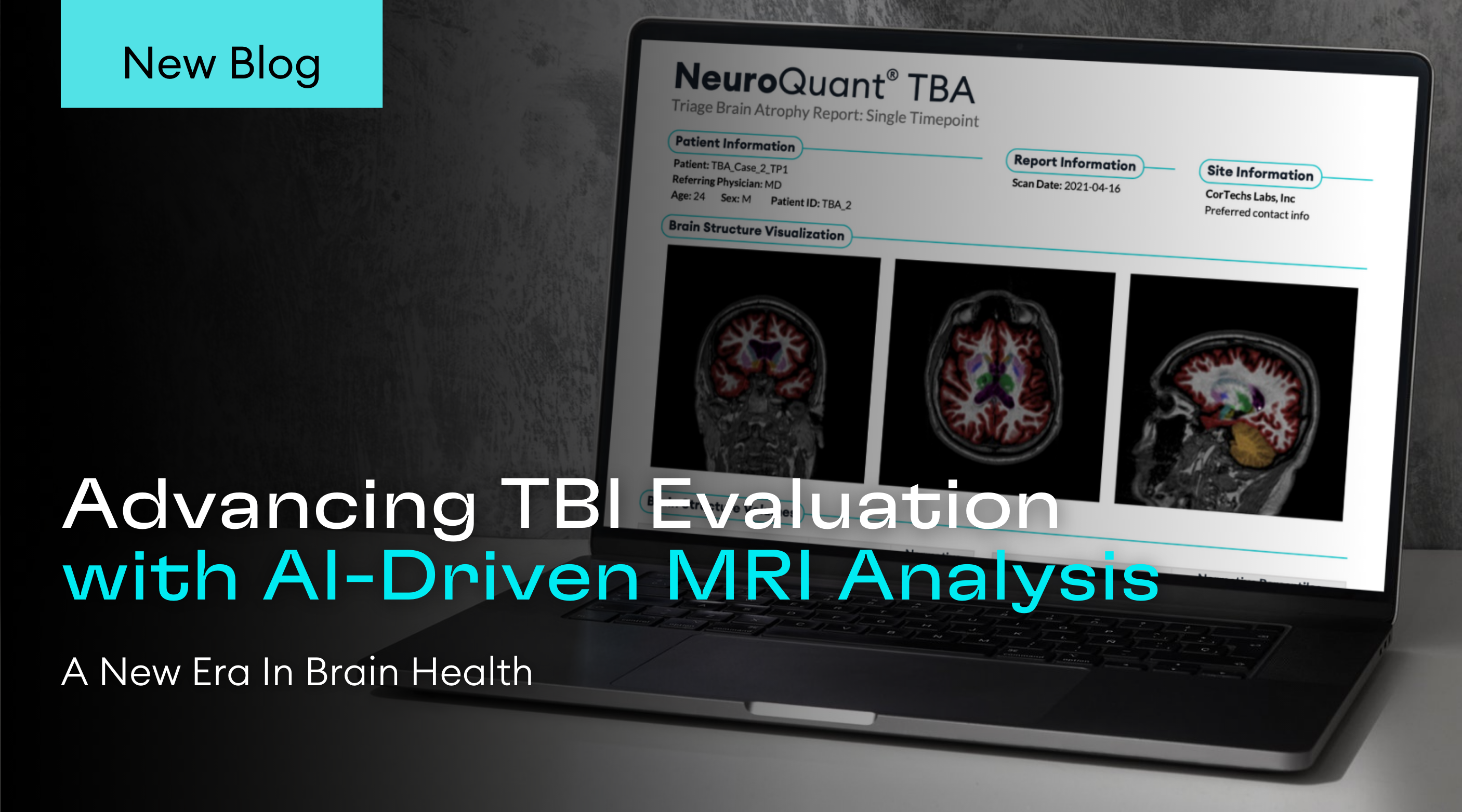
For over 20 years, volumetric MR imaging (vMRI) technology has been available in research and clinical environments.Initially, radiology centers developed and used their own imaging parameters and manually evaluated the resulting images. This made clinical application subjective, inconsistent from patient to patient and from scanner to scanner,and all but impractical. The introduction of large-scale multi-institutional research studies, such as ADNI (Alzheimer’s Disease Neuroimaging Initiative) in 2004, initiated the streamlining and standardizing of acquisition protocols. In 2006, CorTechs Labs introduced NeuroQuant, the first FDA cleared automated segmentation software, which provided consistent, operator independent measurements of brain structure volumes. Together, these factors permitted clinical users to easily incorporate volumetric MRI technology into their clinical practice.